Nitrates and nitrites are compounds that contain nitrogen and oxygen.
Nitrates have three oxygen atoms bonded to the nitrogen, while nitrites have two. Nitrate Nitrates are both the salts and esters of nitric acid. In the biosphere and hydrosphere, nitrates are ubiquitous mainly as sodium nitrate. Autotrophic bacteria convert ammonia to nitrite and then to nitrate under aerobic conditions. When oxygen is not available under anoxic conditions, bacteria can use the oxygen in the nitrate and reduce it to nitrite. Nitrite Nitrites are the salts and esters of nitrous acid.
Nitrite ions are formed by the chemical reaction of nitrous gases with oxygen and moisture in the air (see acid rain) and in the soil in water bodies and in sewage treatment plants by nitrite bacteria (Nitrosomonas) through the oxidation of ammonium ions with the consumption of oxygen. In protein degradation, they are the intermediate product in the complete oxidation of ammonium nitrogen to nitrate (nitrification). They are also formed under anaerobic conditions by bacterial reduction from nitrate ions (nitrate reductase).
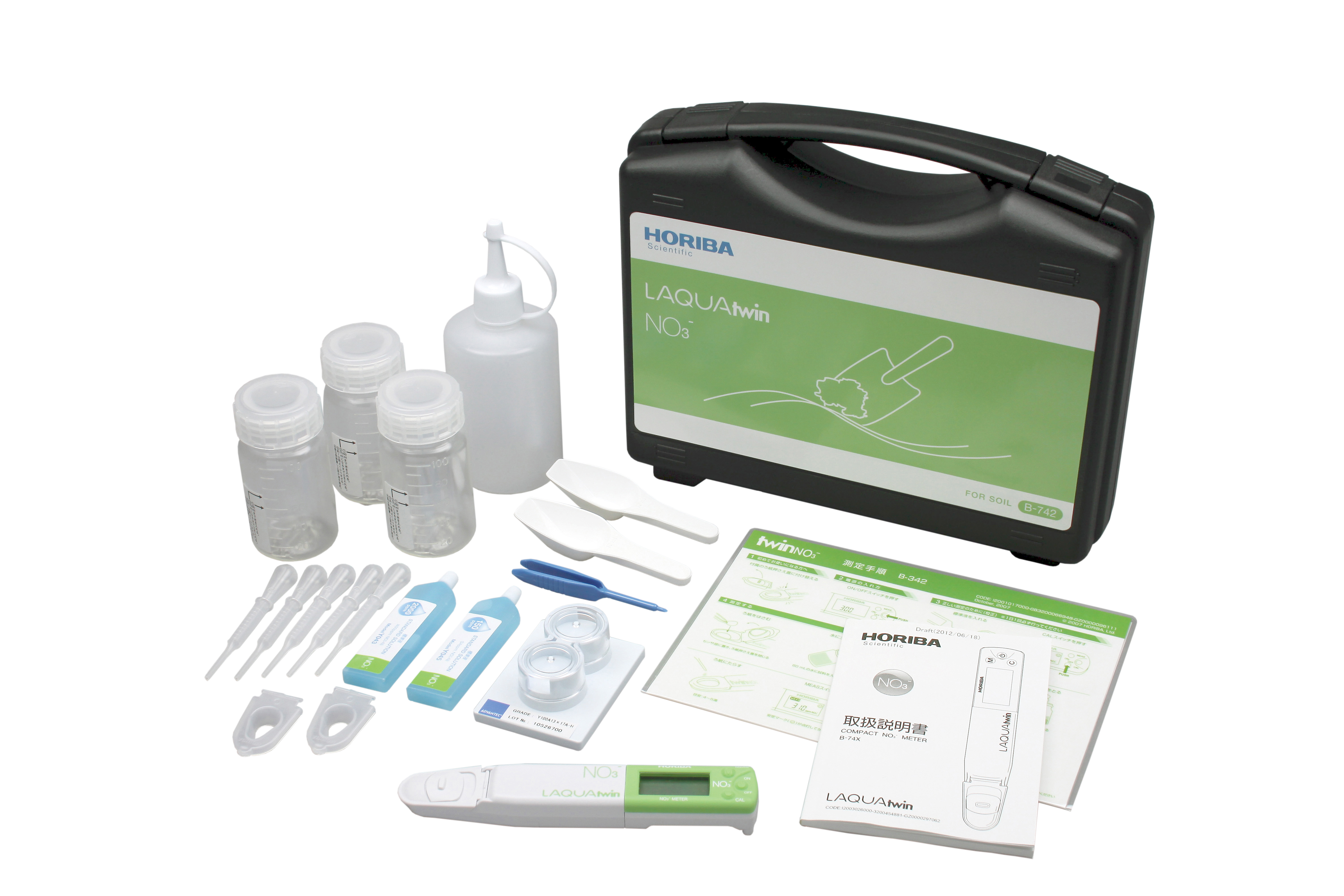
HORIBA LAQUAtwin Nitrate Meter
- All Products
-
Parameter
- pH
- ORP
- Conductivity and TDS
- Dissolved oxygen (DO)
- Combined - Multiparameter
- Ammonia (NH3)
- Calcium Ion (Ca2+)
- Chlorid (Cl-)
- Iron (Fe)
- Fluoride (F-)
- Free Chlorine
- Total Chlorine
- Gloss
- Iodine (I)
- Potassium Ion (K+)
- Seawater specific gravity (SSG)
- Sodium Ion (Na+)
- Sodium Chloride (NaCl)
- Nitrate Ion (NO3-)
- Salinity
- Turbidity
- Applications
- Application examples
- Quality
- Brand
- Buffer and calibration solutions
- Spare parts and single components
- Plant analysis - sodium, nitrate, potassium, calcium
- Laboratory Benchtop Meters
- Accessories
Filter products